What Best Describes the Electron Cloud Model
Electron cloud is an informal way to describe an atomic orbital. An cloud atom model of electron the.
What Is The Electron Cloud Model Of The Atom Quora
It shows that electrons remain in high-energy subshells.

. The cloud is darkest at the nucleus and lighter farther away representing that electrons are more likely to be found closer to the nucleus than away from it. Unit 4 Test Review DRAFT. Berdasarkan model atom berikut yang merupakan model atom dalton adalah.
In biomedical sciences and is a science writer educator and consultant. The electron cloud model says that we cannot know exactly where an electron is at any given time but the electrons are more likely to be in specific areas. The orbitals are specified by shells and sub-orbitals.
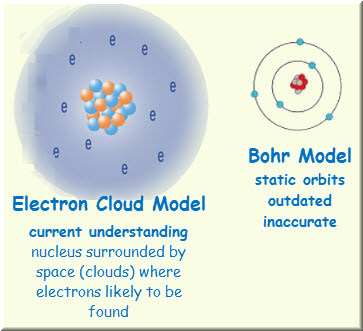
The Electron Cloud Model was of the greatest contributions of the 20th century leading to a revolution in physics and quantum theory. In the electron cloud model of the atom what does the cloud represent. An electron cloud represents the area around an atoms nucleus where electrons are most likely to be found.
The electron cloud refers to a region outside the nucleus where an electron is most likely to be found. Which best describes the electron cloud. The electron cloud shows the area in space where an electron is most likely to be.
Which statement best describes the Lewis dot structure for fluorine. The exact position of an electron can be known. When an atom of lithium loses an electron the atom becomes a.
The probability of the electron location. The cloud is darkest at the nucleus and lighter farther away representing that electrons are more likely to be found closer to the nucleus than away from it. What is the electron cloud model.
These areas are specified by orbitals. In this model of the hydrogen atom you have a black screen and then you draw a. An electron cloud is a visual model of the most probable locations of electrons in an atom.
Which of the following best describes the electron cloud model. The electron cloud model describes the _____ of electrons in an atom. The shape not the size of an electron cloud is determined by the electrons ____.
Location where an electron has the highest probability of being found. Which statement best describes the electrons in this illustration. What is the electron cloud model.
Which statement about the electron-cloud model is true. Understand the Electron Cloud Model. B It is a region in space that has a precise shape and.
The negative charge of the electron. SOLAR SYSTEM MODEL OF THE ATOM. The electron cloud model describes which of the following.
The electron cloud model is a theory of the atom that comports with the modern understanding of quantum mechanics. The group of protons that are in the nucleus. How does the electron-cloud model describe electrons.
An electron has a high probability of being in certain regions. Which best describes an electron cloud. ELECTRON CLOUD MODEL OF ATOM 2 See answers iandinglasagmailcom iandinglasagmailcom Answer.
An electron can be found at certain distances from the nucleus. Helmenstine holds a PhD. The electron cloud is used to describe the behavior of electrons and it is useful in building a model of the atom.
You might also like to know about the parts of an atom before. Location where an electron has highest probability of being. Each electron follows a.
The model dispenses with the classical orbiting electrons depiction and envisions electrons as holding indeterminate positions in a diffuse cloud around the nucleus of the atom. Before understanding what an electron cloud model is it is important to know about the forces that bind the electrons together. What is the electron configuration of this atom.
1s2 2s2 2p6 3s2 3p6 4s1. Which of the following best describes the electron cloud model. It shows that electrons usually carry a negative charge.
Which statement best describes an occupied orbital according to the quantum mechanical model. She has taught science courses at the high school college and graduate levels. BILLIARD BALL MODEL OF THE ATOM C.
The shape not the size of an electron cloud is determined by the electrons ____. This assessment will help you. Erwin Schrodinger an Austrian physicist came up with the electron cloud model in 1926.
An electron cloud represents the area around an atoms nucleus where electrons are most likely to be found. The group of protons that are in the nucleus. The probability function basically describes a cloud-like.
SOLAR SYSTEM MODEL OF THE ATOM B. The trails left by an electron as it moves around the nucleus. Which of the following best describes the modern atomic theory.
The electron cloud model describes which of the following. It shows that electrons move quickly in circular orbits. What best describes the electron cloud.
The group of protons that are in the nucleus. The electron cloud is the location around the nucleus that contains negatively-charged electrons. The electron cloud model describes which of the following.
Intersects With Rectangular Pools. A group of electrons circulating around a nucleus or a molecule. Answered expert verified.
The negative charge of the electron. PLUM PUDDING MODEL OF THE ATOM D. An electron cloud is a visual model of the most probable locations of electrons in an atom.
It shows that the electrons within an atom do not have sharp boundaries. The Electron Cloud Model Of An Atom Till Lindemann Metal Wings. But according to the wave mechanical or cloud concept model the electron keeps on moving away or towards the nucleus and the maximum probability of locating it lies at a distance of 0529 Å from the nucleus.
A It is a spherical or dumbbell-shaped route traced by the electron in its rapid movement. The electron cloud is a theoretical model that attempts to explain the erratic behavior of electrons in an atom.
Sol Ps 2 Properties Of Matter Standards

Nitrogen Electron Configuration Electron Configuration Nitrogen Electrons
No comments for "What Best Describes the Electron Cloud Model"
Post a Comment